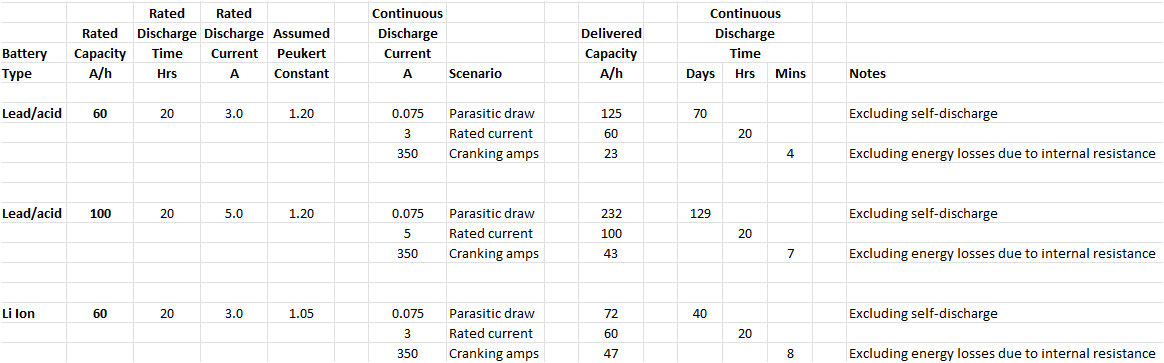
As a follow-up, I've done some quick & dirty calculations for 60 and 100 Ah lead/acid batteries and a 60 Ah Li Ion. Three scenarios are shown for each: 75 mA parasitic drain (typical of a parked & locked modern car), 350 A discharge (guesstimated cranking current for a V8), and a 20 hour discharge (just to verify my calculations - the delivered capacity should then match the rated capacity).
As noted below this takes no account of the natural self-discharge over time that any battery suffers, although this shouldn't be significant here. Energy lost as heat at high currents (due to the internal resistance of the battery) is also excluded, and this would likely affect the results ... reducing the capacity delivered and cranking time shown.
The Peukert Constant values used are also just examples. 1.05 is typically quoted for Li cells, but Lead/acid batteries have quite a range (AGM is actually very similar to Li, gel is in the middle, and wet is the worst). As mentioned they are all affected by ambient temperature and the age of the battery. Of course Li batteries will be significantly smaller and lighter (but more expensive) than any type of lead/acid of similar rated capacity.
With these points made, for continuous engine cranking a 60 Ah Li battery seems to out-perform (just) a 100 Ah lead/acid. But at very low currents (parked car scenario) even a 60 Ah lead/acid will last a lot longer. I suspect exotic ICE cars with Li starter batteries are intended to be kept on a maintenance charger if not driven regularly?
If you'd like to see the results with different values for the Peukert Constants or other battery capacities I can easily do that.
View attachment 158904